The
largest plant after 21 days growth.
Organisations
directly involved:
ARESA
Biotech - trial originators and the Client [ARESA]
MgM (Menschen gegen Minen) - Host and site-supplier [MgM]
AVS Mine Action Consultants - Observer and Advisor [AVS]
Contents
The
independence of the author
Summary
Aim and scope
Authority
Constraints and limitations
Trial format
Criteria
Execution
Conclusions
Recommendations
Annex A: ARESA pre-trial instruction and risk assessment
Annex B: TRIAL INSTRUCTION - ARESA "development trial" - AVS,
Sept/Nov 2003
Annex C: Pre-Trial Assessment - Andy Smith 30/09/03
Annex D: AVS/ ARESA Biotech contract
Annex E: The MgM detector test-area
Annex F: Angola ARESA development Test diary - AVS 2003
The
independence of the author
The
role of Andy Smith (AVS) was described in his contract with
ARESA Biotech as:
Observer:
AVS shall document the experiment carried out in Angola by writing
up a report that describes the test results achieved. It is
of high priority that the report emphasises on the sensitivity
of the plant line(s) grown in respect to the different type
of mines detected/not detected. Furthermore, the report shall
contain reflections upon observations made in respect to the
practical use of the technology (seeding, watering techniques
and systems, water requirements, plant growth, etc.) in light
of the complexity when operating in a landmine infected area.
Technical
advisor: AVS shall act as an advisor on the technology in
development on behalf of his in-depth experience within de-mining.
Hence, AVS shall challenge existing ways of thinking, and discuss
new ideas generated with the employees of the company.
Safety
advisor: AVS shall act as an advisor on safety related issues
due to his experience from operating in landmine infected areas.
Hence, AVS should guide the employees of Aresa and employees
of Bastard [the film company] to behave as safely as possible in Angola. In case
of any doubt, AVS should consult the MGM safety Officer Ken
O'Connell for advice on safety related matters.
The
full contract is reproduced at Annex D.
Disclaimer
Andy Smith (AVS) was retained to produce
an independent report on all the ARESA tests, trials and data-gathering
exercises conducted at the MgM camp in Ondjiva, Angola during
October and November 2003. As an "Observer" and advisor, AVS
had no control over the content and conduct of the trials. Further,
AVS declares that he has no "interest" in the outcome and so
was able to apply disinterested and objective observation overlaid
with opinion and advice. The fact that AVS has responded to
requests to offer advice and opinion (recorded in this document)
does not imply any acceptance of responsibility for the conduct
of the tests/trials, the materials used, or any consequences
(legal or otherwise) arising for the activities of ARESA Biotech
or MgM in Angola or elsewhere.
AVS
reserved the right to withdraw from his contract if activities
that he considered to be physically or ecologically irresponsible
or unsafe occurred.
Summary
Responsibility
for the detailed aim and scope of the ARESA "development trial"
rested entirely with ARESA Biotech. Although they were advised
on some issues by AVS and MgM, there was no obligation for them
to heed advice. I prepared a formal "Trial Instruction" based
on ARESA documents, reproduced at Annex B. However, ARESA had
not described the trial aims until shortly before my departure,
and so did not receive the Trial Instruction in time to take
notice of it before they deployed.
MgM
provided a dedicated detector test-area with appropriate facilities
(detailed at Annex E), including a clean potable water supply
and some staff assistance. MgM provided further site facilities.
The
conduct of the trial was such that I resigned from my contract
after 24 days (14 days at the site). At that time, I advised
MgM that it was not possible to safely halt the trials without
allowing the seeded plants to grow so that they could be seen.
[No means of destroying seed that not not yet germinated was available.} MgM made my continued presence a condition of the Trials' continuation,
so I felt obliged to remain as unpaid observer. I did so with
enhanced authority to prevent further spreading of GM material
outside the test area.
Ambient
conditions combined with the methods of seed dispersal combined
to limit plant growth and the Trial failed to realise any of
its stated aims. It was halted prematurely by the decision of
ARESA staff to leave when the BASTARD television crew were unexpectedly
obliged to leave by the apparent hostility of the authorities.
ARESA staff left the area in haste, leaving GM material. AVS remained
for a further seven days, watering the test area and tending it as required by ARESA. No
further significant growth having occurred, I then (by agreement
with ARESA) destroyed the stunted growth and made the site as
"safe" as possible (within the constraints of my limited knowledge
of biotech safety). [In addition to the inadequate weedkillers left by ARESA, I used a gas-torch to burn off the area and its surrounds, including dry areas with no growth where ungerminated seed was likely to rest.]
1
Aim and scope
The
aim of the ARESA development trial can be summarised as follows.
A)
To establish whether the genetically modified plants (known
as A line: Genotype PAP1/tt4/nii-CHS/ga1-3/ExenA (designated
LA); B line: Genotype PAP1/tt4/nii-CHS/ga1-3/ExenB (designated
LB), change colour when grown above hidden landmines and UXO
in the test area, and to assess the reliability of any colour
change that does occur.
B)
To establish whether the genetically modified plants produce
flowers.
C)
To determine the efficacy of the proposed seed distribution
system and reach a view on whether it and its ancillary parts
have the potential to achieve field utility.
D)
To determine whether either of two flowering genetic variants
have the same or better potential than the main (LA and LB)
variants by reference to their growth in the prevailing conditions.
E)
To devise and implement a watering regime on unmodified plants
to determine the minimum acceptable water requirements and also
the requirement that is optimal for fast growth.
F)
To gather data to assist in further ARESA research.
G)
To gain some field experience of the way in which Humanitarian
Demining is controlled and conducted.
H)
To facilitate the recording of a video record of the testing.
The
Trials took place over a period of 25 days at the dedicated
MgM detector test area in Ondjiva, Angola.
1.1
Authority
The
relevant Authority for the trial was the provincial Governor
(Cunene) and the Department of Agriculture in Angola. A further
authority was MgM, the host who assisted with the requesting
of permission from the relevant authorities and imposed some
of their own constraints on ARESA activities.
Those
involved in the trial were:
ARESA
Biotech - trial originators and the Client
AVS
Mine Action Consultants - Observer and Advisor
MgM
(Menschen gegen Minen) - Host and site-supplier
ARESA
Biotech assumed full responsibility for the design of the trials
and the day to day conduct of data-gathering and plant care.
A TV documentary crew from the Danish company BASTARD accompanied
ARESA personnel to the site and made a record of what occurred.
Filmed interviews about the testing were made with the permission
of the senior ARESA staff member (Carsten Meier) who had ultimate
authority over what went on the public record. Carsten Meier
for ARESA placed no restrictions on the interview content. MgM had editorial rights over the footage eventually used, so I responded frankly to questions from the TV crew. [The TV crew subsequently reneged on its agreement to give MgM editorial rights. Ed.]
1.2
Constraints and limitations
Constraints
intended to be appropriate for the testing of genetically modified
material were adopted by ARESA in their pre-trial documentation
(see Annex A).
Prior
permission for testing GM plants was sought from the Provincial
and National Authorities. The request to the National Authorities
was made less than two weeks prior to the trial's start date.
It was known that Angola is a signatory to a SADEC treaty limiting
GM crops and produce, so it was considered unlikely that the
Government would approve the trial. Permission was granted at
Provincial level and no direct denial of permission was received
from the National Authorities. No formal response to the request
to hold the trials had been received from the Angolan National
Authorities at the time the trial finished.
ARESA
personnel, myself and associated visitors to the MgM site were
obliged to obey MgM camp rules and while staying in the area
were requested only to travel in company with MgM representatives
or after seeking prior advice.
The
greatest predictable constraint placed on the trials was the
perceived risk of the escape of biologically modified
material into the environment. A second major constraint was
the need to carry out experiments in areas where landmines had
been concealed for a long period of time.
1.2.1
Unanticipated constraints
The
trial was constrained by the pre-prototype design of ARESA equipment
brought to the site and by the fact that it had not been fully
assembled and/or tested prior to its arrival. Examples were
the seed-distribution method, insect-proof netting cloches,
area-marking signs and the watering systems.
The
fact that the netting was not "pollen proof" was a surprise
because ARESA management had previously used that expression
to describe it. This may have been a simple communication difficulty
caused by their use of English as a second language. The pollen
is far smaller than the anticipated insect threat and could
be wind-borne through the netting used. This increased concern
over the escape of pollen if the plants were allowed to flower.
1.3
Trial format
The
trails took place over a 25 day period. By request, I was on
site for one week after the ARESA staff left, and had been engaged
for 10 days prior to the formal start of the trial in the test
area.
By
agreement, the positions and identity of targets concealed in
the test-area was made known to the data-gathering team.
Details
of the prior preparation of the lanes containing test-targets,
their sifting, drainage and depth are in Annex E along with
details of the targets, their depth and their positioning.
Although an ARESA staff member had visited the site once before,
most aspects of the site conditions required variations to be
made to the pre-trial planning. Perhaps most obvious of these
was the fact that all tests involving GM and mutant seeds occurred
within the double-fenced detector-test area (the dog-training
area was not used).
ARESA
took sole responsibility for the conduct of the trial, variations
to its objectives and the methods of gathering data. I advised
constantly, as detailed in Annex F.
MgM
retained sole responsibility for safety aspects, excluding those
pertaining to any biological hazard resulting from the test
materials and with the proviso that all visitors acted at their
own risk. No MgM staff member was assigned to the team, so that
safety relied heavily on heeding advice and applying self-discipline.
1.4
Criteria
I
was not asked to develop trial criteria. To develop detailed
judgement criteria without greater knowledge of biotechnology
would not be appropriate. However, I was asked to comment on
the outcome and will do so with reference to the following:
1.
A perceived change of colour occurring above buried ERW.
2. The consistency of any colour change with reference to the
specific ERW beneath.
3. Whether or not the G3 modification flowered.
4. Whether any random colour changes occurred without the presence
of ERW.
5. The practicality of the deployment method in the field.
6. The apparent professionalism with which the trial was conducted
and records were kept.
7. A subjective judgement about the potential utility of the
technology.
Methodology,
evaluation and acceptance criteria were determined and judged
internally by ARESA, but are commented on by me when appropriate.
I advised on all aspects on which I felt qualified to comment
and some of that advice is recorded in this report.
2.
Execution of the Trials
The
following is a simplified Trial schedule:
AVS
left for South Africa in pursuit of a visa on Sunday 28th September
AVS arrived in Namibia on Saturday 4th October
AVS arrived in Ondjiva on Sunday 5th October and began pre-trial
preparations.
ARESA and BASTARD staff arrived at the site on Wednesday 8th
October
Seeds were first spread on Sunday 12th October
AVS resigned from his "Observer" contract with ARESA and became
site "policeman" and QA on Monday 20th October
ARESA and BASTARD left the site on Monday 27th October
AVS left the site after "cleaning up" on Tuesday 4th November
A
record of the events during the trial is available.
The
trial was brought to an end by the decision of ARESA staff to
leave when it became clear that the BASTARD film crew were no
longer welcome. The
ARESA staff decided to leave with the film crew. By that time
the failure of the plants to grow was obvious, leading me to
conclude that ARESA took advantage of an "excuse" to leave so
that the trials would be "unfinished" rather than a failure.
The ARESA staff said that they left because they also felt under
some threat from the local authorities. The reported threat
was only to the film crew. The Provincial
authorities showed no apparent interest in the activity at the
test-area after the ARESA and BASTARD personnel had departed.
2.1
Preparation
ARESA
preparation was generally inadequate. Examples are:
The
varied seed brought by ARESA was not prepared in the way that
was documented in their pre-trial papers. The GM seeds were
mixed together and had not been coated or "pelleted". Coating
the seeds made them large enough to be seen and so relatively
easy to disperse evenly. Uncoated seeds were a fine dust that
was readily wind-borne and so were widely dispersed unintentionally.
The entire conduct of the trials was affected adversely by ARESA's
failure to bring appropriately prepared seed.
The
schedule was constrained by the late arrival of a box of equipment
that was only available to be unpacked on Sunday 12th October.
It did not contain everything that AVS had been told it did,
which implies that the ARESA staff did not know what had been
packed.
Tools
and equipment used by ARESA were not appropriately "scientific"
to allow them to achieve the aims stated in their pre-trial
documentation.
2.2
Achieving the aims
It
was not possible to achieve the stated aims for a variety of
reasons. The aims and the results are summarised below.
A)
It was not established whether the genetically modified plants
changed colour when grown above hidden landmines and UXO in
the test area. At the end of the Trials, AVS was told that the
root needed to reach a length of 20cm in order for a colour
change to occur. The longest root was under 20mm in length at
that time. The seed from which that plant has grown had been
planted on 12th October (it was re-seeded on 21st but the largest
plants presumably came from the first seeding). The Trial ended
on 2nd November so that the plants had 21 days to grow. The
scientists had expected the plants to have matured within that
time-frame, achieving a height above ground of around 15cm.
At the end of the Trial, the above ground height of the largest
plant was not more than 3mm.
B)
It was not established whether the genetically modified plants
would produce flowers. Pre-trial assurances that the genetic
coding had been modified with what was nicknamed a "suicide-gene"
were varied when ARESA was on site, and their scientist announced
that the length of the day might over-ride the "suicide-gene".
The day length was well under 14 hours from dawn to dusk (and so quite predictable).
C)
It was determined that the seed distribution "systems" tried
with the GM seeds were either unsafe and/or failed to achieve
an adequate seed distribution. They also failed to achieve distribution
of seeds that had been suitably affected by the growth hormone
with which they were supposed to be "activated".
The spray distribution of pelleted seeds (using a compressor
and water/seed spray mix imported by ARESA) did distribute seeds
fairly evenly over a ten metre box (with some overspray of seeds
which flew further than the water). Despite being unmodified
and pelleted seed, none of this seed grew. It is not possible
to determine whether the distribution system played any part
in that failure.
The use of a DriWater slurry to aid dispersal was tried without
success. DriWater is a powder that, added to water, forms a
transparent slurry that is physically akin to wallpaper-paste.
It is intended to provide a "skin" over the ground and limit
evaporation. Failure may have been because the growth hormone
was added after the dispersal of seed in DriWater, and so the
hormone did not reach the seed.
Although the DriWater was still wet on application of the hormone,
its gelatinous nature seems likely to have prevented contact.
Alternatively, the method of applying the hormone may have limited
its distribution. It was applied mixed with water and using
a bucket with holes in the bottom. A later, second application
of hormone appeared entirely ineffective.
D)
It was not possible to determine whether either of two flowering
genetic variants had the same or better potential than the main
(LA and LB) variants by reference to their growth in the prevailing
conditions because they were not grown under the same conditions.
The flowering variants were grown under white "insect-proof"
netting which provided (unmeasured) shade and UV protection.
The seed was dispersed over a much smaller area that had been
"ponded", preventing seed dispersal. The plants appeared more
quickly, but did not appear to grow any larger than the GM variety.
The flowering plants were hoed out of the ground by ARESA staff
when hurrying to make their premature departure, so AVS was
unable to measure root length.
E)
It was not possible to devise and implement a watering regime
on unmodified plants to determine the minimum acceptable water
requirements and also the requirement that is optimal for fast
growth. This was because, no matter how much water was applied,
the plants appeared reluctant to grow. The ARESA presumption
that water availability would be the most significant growth
factor appears to have been erroneous.
F)
It is not possible for AVS to determine how much data of assistance
to further ARESA research was gathered. However, the absence
of any scientific instruments to record the context of the trials,
and the failure to constrain and record variables, must mean
that any data gathered is of questionable utility.
G)
ARESA staff did gain some field experience of the way in which
Humanitarian Demining is controlled and conducted, but very
little.
H)
The aim to facilitate the recording of a video record of the
testing was not achieved despite the presence of the BASTARD
television crew because all film and associated records were
confiscated from them as they left the country (or so they subsequently reported).
The
more scientific aims could not be achieved because appropriate
instrumentation to record the context of the trial was not brought
by the ARESA team. The more obvious failings in this regard
are listed below:
1)
No means of measuring temperature or humidity was on site (an
AVS thermometer measuring up to 50C was borrowed, but ground
temperature regularly exceeded 50C).
2)
The means of measuring soil-moisture utilised two cheap gardening
prods that measured electric resistance between probes and used
a scale that was not understood. Without an analysis of other
soil properties, they gave only the crudest indication of moisture
content. The probes were left behind at the site, so preventing
any later comparison of their results with another instrument.
3)
No means of measuring wind-speed was on site. Blustery wind
affected dry seed dispersal significantly.
4)
No means of measuring UV intensity was used. This meant that
the influence of the shade-netting brought (and second type
purchased locally) could not be measured.
5)
The compressor, water tank and spray lances brought by ARESA
were not much used, apparently because varying the pressure
was not understood, Near the end of the trial ARESA informed
AVS that pressure could (after all) be varied. Similar equipment
had not been assembled and tested prior to dispatch, which meant
that its strengths and limitations were not known. This equipment
was not necessary unless a mixture of pelleted seed, Driwater
and growth hormone were to be dispersed. The failure of ARESA
to bring pelleted GM seed made the equipment largely redundant.
6)
No soil samples were taken to allow later analysis of its properties.
2.3
Safety
The
trials were conducted in a manner that probably dispersed dry-dust
seed outside the test area. The trials were also conducted in
a way that allowed insects and birds access to the plant heads
as they emerged from the soil, and the presence of both insects
and birds around the plants (apparently eating) was observed.
It is not certain that the birds ate the plants, but the fact
that they were attracted to the new growth makes this likely.
Bird scarers (ineffective) were only erected at my insistence
(after resigning from the ARESA "Observer" contract). The shade-netting
(potentially bird-proof) was not kept sealed by ARESA personnel.
ARESA
disposed of utensil "washout" water in two pits - so mixing
varied seeds and growth hormone in the same small areas. AVS
marked the first pit (that had been concealed) and later burnt
both pits out using a gas-torch. The effectiveness of this could
not be measured.
ARESA
did not provide a "pollen-proof" netting for the "flowering"
plants as described in their pre-trial documentation. The netting
was nominally "bug-proof" but could be watered through and was
rapidly torn in places (in any case it was placed on top of
bugs in an area where bugs lived below ground). The failure
of the plants to grow to any significant size means that there
were no obviously adverse safety consequences as a result of
ARESA's failure to provide the protection planned.
The
"weed-killer" provided by ARESA and used by AVS during the clean-up
of the site was not of a "known" safety. The edge of the site
is within 50 metres of the MgM camp's potable water borehole.
I repeatedly asked ARESA to check the safety of using their
locally purchased weed-killer (branded "KOMBAT Wipe Out") with
regard to its long-term ground contamination. This request for
assurance was repeated to ARESA management in Denmark (seen
Annex F). No response was ever received. The weed-killer is
"a water soluble concentrate non-selective, foliar, selective
herbicide for the control of a wide range of annual and perennial
grasses, broadleaf weeds and certain woody perennials". Its
active ingredient is "Glyphosate timesium (suffosate) at 480g/l".
The
area in which I used the weed-killer was more than 120 metres
from the borehole, which reportedly draws water at a depth of
40 metres. Without suitable advice from ARESA, I was obliged
to make an uninformed risk-assessment and decided to use the
weed-killer. Any seed that may still germinate will not be affected
by the weed-killer.
I
used a gas-torch, a hoe and weed-killer to destroy all visible
plants and burn the top of the soil in areas where seed was
known to have been planted. [All damage limitation of this kind
would not not been done without my
independent decision to do so.]
3
AVS Comment
I
was asked to comment on the outcome with reference to each of
the following topics. The topic is repeated and a comment appended:
1.
A perceived change of colour occurring above buried ERW.
This did not occur - possibly because the plants did not reach
maturity. However, an 18mm root length did mean that the roots
were in proximity to (beneath) at least one concealed device.
2.
The consistency of any colour change with reference to the specific
ERW beneath.
There was no colour change to be consistent.
3.
Whether or not the G3 modification flowers.
No plants approached a stage of maturity at which they could
have flowered.
4.
Whether any random colour changes occur without the presence
of ERW.
No colour changes occurred, random or otherwise.
5.
The practicality of the deployment method in the field.
The deployment methods used (including those contrived on site)
were entirely impractical for use in or near a genuinely hazardous
mined area.
6.
The apparent professionalism with which the trial is conducted
and records are kept.
The conduct and recording of the trials did not appear professional
and did not lead to the acquisition of any accurate scientific
data.
7.
A subjective judgement about the potential utility of the technology.
I believe that the concept has value, but the plant type used
was adopted for expediency (its genome was already known and
in the public domain) and the particular plant is not appropriate
for the exploitation of the concept. Successful exploitation
would require the development of an appropriate slurry dispersal
system (including all water, nutrients and seed) ensuring that
the plants grew anywhere. Another prerequisite is that the type
of plant be hardy enough to grow in most areas of the world.
I
had reserved the right to withdraw if activities that I considered
to be physically or ecologically irresponsible or unsafe occurred,
and I was obliged to withdraw part way through the Trial. By
negotiation, I remained as Observer and QA
with enhanced authority to ensure that ARESA gave appropriate
weight to safety concerns.
In the contract between AVS and ARESA, the following confidentiality
constraint is detailed:
In
order to control and protect the patent strategy and the communication
strategy of the company, AS agrees to treat all knowledge and
insight material regarding the use and the development of the
technology platform, which may come to his possession, as strictly
confidential. Hence, AS will only communicate any information
about the company and its technology to a third party, after
getting accept from the management of the company.
I
(AVS or AS) have acquired no "knowledge and insight material"
regarding the use or supposed development of the "technology
platform" (GM cress), so I am not in any position where I could
either wilfully or inadvertently breach this confidentiality
agreement.
The
events and conduct of the trial are not covered by this agreement
- and I was expressly required to record them with objectivity
and honesty. Accordingly, I reserve the right to comment on
the events and conduct of the trial as documented herein whenever
appropriate. I would consider it (professionally) appropriate
to do so if the events of the trial were publicly misrepresented
by any other party.
4
Conclusions
A "development
trial" is defined as a trial seeking to establish whether the
concept under assessment has the potential to achieve its stated
aims and whether any materials involved are fundamentally flawed
in a manner that would render it unusable in the field. It is
not possible to state definitively whether the concept under
assessment has potential, but it seems evident that the means
of applying the concept (European water-cress) has no potential
in the prevailing conditions in Ondjiva (those conditions were
not recorded).
The
trials were conducted in a manner that did not guarantee that
genetically modified seed and material failed to enter the wider
environment and the food chain.
The
Trials did not fully achieve any of the stated aims. There were
a number of identifiable reasons for this:
1) Inadequate
preparation of seed.
2) Inadequate lab-preparation under simulated conditions.
3) Inadequate pre-trial planning (including the planned use
of "controls").
4) Inadequate pre-trial testing of both seed and equipment.
5) Failure to deploy appropriate means of recording local soil
and weather conditions.
6) Failure to react in a timely way to local conditions (shade-netting
and plant-feed).
7) Failure to ensure the legality of the testing prior to deployment.
8) Failure to deploy appropriate clean-up equipment.
Looking
at the details of the results anticipated in the ARESA pre-trial
instruction and risk assessment (See Annex A) it is inexplicable
that ARESA should have failed to bring as much instrumentation
as a thermometer with them. The cost of the trial must have
been high, and much of that cost could have been avoided if
sensible preparation had been made. That preparation would undoubtedly
have led to a delay, which would have had two advantages. The
Angolan government authorities would have had time to reply
to the request for the trial to take place. And the seed used
could have been appropriately prepared (as ARESA had always
intended it should be).
The
only real result from the trials is the conclusion that the
use of a Genetically Modified European watercress for the purpose
of identifying concealed TNT and RDX in Southern Angola did
not work at all, because the plant would not grow in the (unrecorded)
conditions prevailing at the test centre. Lessons to be derived
from this are severely limited by the failure to record local
conditions and the uncontrolled (and so unrepeatable) methods
of seed mixing and dispersal. It simply is not known how the
(apparently) infertile soil, its rapid drainage or the UV intensity,
ambient temperature, or random seed dispersal and inefficient
application of the growth hormone affected these trials.
5.
Recommendations
I
have no recommendations to make to ARESA, who have expressed
no interest in receiving this report. MgM should check the test-area
for plant growth every month for six months, and destroy by
burning any of the plants that appear.
MgM
should have the camp's water supply subjected to chemical analysis
(checking for "suffosate" or derivative traces) at three month
intervals until confident that no contamination has occurred.
If contamination is present, another potable water source should
be found.
MgM
should not consider allowing similar trials to take place without
first gaining the permission of the appropriate National Authorities..
Annex
A: ARESA pre-trial instruction and risk assessment
Reproduced
from an original *.pdf file received on 26/09/03.
Comments
in square brackets [ ] are additions made by the author of this
report.
A R
E S A B I O D E T E C T I O N A p s
S Ø L V G A D E 1 4 A ~ 1 3 0 7 C O P E N H A G E N K
T E L : ( 4 5 ) 7 0 2 2 7 7 4 7 ~ F A X : ( 4 5 ) 7 0 2 2 7
7 5 7
M A I L : i n f o @ a r e s a . d k ~ W E B : w w w . a r e
s a . d k
Description
and risk assessment of the landmine detection test carried out
by Aresa Biodetection at the test site of MGM in Ondjiva, Angola
This
document shall provide information and insight to the testing
performed at the test side of MGM in Ondjiva, Angola for a period
of five weeks beginning on the 8th of October, 2003.
Aresa
Biodetection (hereafter: Aresa) is a new player within the de-mining
community. The company has several years of experience within
molecular and physiological studies of plant development. The
ideas behind the technology developed by Aresa grew out of The
Department of Plant Molecular Biology at the University of Copenhagen.
This research group, and thus the employees, is famous on an
international scale for its scientific findings. In order to
combine the biotechnological technology of Aresa, the company
has signed up with Menschen Gegen Minen (MGM) to carry out the
first testing of the plant lines developed when growing these
in the presence of real landmines at the test site in Ondjiva,
Angola.
This
test is of utmost importance for the further development of
the plant technology of Aresa, which eventually shall contribute
to the removal of landmines in Angola and other landmine infected
areas. Therefore, Aresa is grateful to MGM for contributing
to the research by making it possible to test the developed
plant lines in the test lanes containing landmines, and to perform
other relevant tests related to the application of the technology.
Furthermore, Aresa is also grateful to the Provincial Governor
Mr. Mutinde, who has approved the use of the test site for growing
the genetically engineered plants at the test side of MGM.
The
Chief Technology Officer Carsten Meier will be in charge of
any decisions to be made at the test site in Ondjiva, Angola.
Carsten Meier will act as the point of contact in Angola regarding
the testing, and Simon Østergaard (Managing Director, Aresa)
will act as the point of contact in Denmark.
This
document will describe the experiments designed, as well as
the purpose with these experiments. Furthermore, a thorough
risk assessment on the use and the release of the genetically
engineered plants in the test site is carried out in a scientifically
sound manner with the conclusion that Aresa is confident in
the performance of the outlined test - with full attention on
reducing and controlling any risks identified before hand and
during the testing. Hence, it is ensured that development, handling,
transport, and the release of the genetically modified plants,
as well as the clean-up procedure after the testing, are undertaken
in a manner that prevents or reduces the risks to biological
diversity. All types of seeds (genetically engineered seeds
and non-engineered seeds (mutant seeds and different ecotypes))
transferred from Denmark to Angola via Namibia, will be packaged
and marked separately at the University of Copenhagen, in order
to achieve the safest handling of the seeds as possible.
Content
The
technology platform in brief
The
test in Angola:
1. Detection of landmines in the 7 test lanes
2. Seed spreading and maintenance of growth using the water
pump system
3. Seed density test
4. Background physiological measurements
5. Examination of the herbicide concentration on local plant
growth
6. Growth experiments with different ecotypes
7. Growth experiments with different mutants plant lines
8. Growth control experiments with different genetically engineered
plant lines
Risk
assessment of the field trial:
1. Information about Thale cress (Arabidopsis thaliana)
2. Why is it necessary to follow up the trials under closed
conditions in the greenhouse with trials in open conditions?
3. Description of the inserts (transgenes)
Environmental
risk assessment:
1. Which non-target organisms could possibly be effected?
2. Prevention of the spread of genetically modified material
in the course of this study
3. To which plants could the transgenic Arabidopsis conceivably
transfer its genetic information?
4. Additional genetic markers were introduced to the plant lines
along with the genetic origins of interest
5. Assessment of antibiotic resistance
6. Assessment of herbicide resistance
7. Deficiency in production of the growth hormone gibberellin
(ga1-3 mutation)
8. Scientific background for the ga1-3 mutation
References
Declaration
of the content of this document
The
technology platform in brief
The
technology platform of Aresa is based on genetic engineering
of the plant Arabidopsis thaliana. This plant is a widely used
genetic model system, and the plant is part of the natural flora
all around the world. The plant has been engineered in order
to respond to specific outer stimuli present in the environment
resulting in a highly visible colour change of the plant within
3-4 weeks if the system is triggered. Thus, the genetically
engineered plant serve as a bio-detection system able to monitor
specific compounds of interest. Using this technology platform
for de-mining, the plant line will react on NO2-groups, which
are cleaved off from the explosive molecules that leak out of
the buried landmine. Consequently the triggered colour change
of the plants will occur in the vegetative stage of plant growth,
which enables identification of the specific plants growing
in the near by presence of landmines within 3-4 weeks.
The
genotypes of the specific transgenic plant lines are shown in
appendix A. [Appendix A was not made available.] The different
functions of the genetic components that are engineered or introduced
by traditional crossing in comparison with wildtype plant lines
(such as different ecotypes) are briefly explained below:
CHS:
The gene that is responsible for production of Chalcone Synthetase,
which enables biosynthesis of the flavonoid derived pigment
anthocyanin (red colour).
Nii:
The regulative element (the promoter) that is fused with the
CHS gene. Thus, the regulation of the Nii-promoter is responsible
for expression of the CHS gene in the presence of NO2-molecules.
PAP1:
Transcription factor involved in overproduction of Chalcone
synthetase.
tt4:
Mutation in the original gene encoding Chalcone Synthetase.
This mutation serves as a null-genetic background where no Chalcone
Synthetase is produced, unless the production is triggered via
the Nii-CHS construct.
ga1-3:
Mutation in the GA biosynthetic pathway responsible for gibberellin
production, which makes the plant unable to germinate and to
set seeds making the plant male sterile.
ExenA/ExenB:
Bacterial reductases that cleave the NO2-group of explosives,
and by then, enhance the sensitivity of the genetic system.
The
test in Angola
The
test that will be carried out in Ondjiva will include growth
of the genetically engineered plant line in the test lanes containing
landmines, as well as a number of growth experiments on different
ecotypes or mutants plants. It is obviously very important to
investigate how suitable are the current plants for detection
of landmines, but it is also of great value to examine a variety
of physiological parameters. The latter is important since the
application of the technology should be based on an ecotype
that is able to grow optimally under the growth conditions of
Africa. The goals and the test procedures are described below:
1. Detection
of landmines in the 7 test lanes
Goal:
To test the genetically engineered plant lines. It is of utmost
importance to test the mixture of seeds from the two specific
plant lines that contain all the several traits introduced (the
genetic cassette responsible for the colour change, reductases
that cleave explosives, and gibberellin deficiency). The sensitivity
of these two specific plant lines will be explored by growing
these homogenously in the test lanes and then observing the
expected colour change caused by explosives of the buried landmines.
Procedure:
The 7 test lanes are grown with a mixture of pelleted seeds
of plant lines LA and LB (the two plant lines only differ in
the specific reductases introduced (ExenA/ExenB)).
A line:
Genotype PAP1/tt4/nii-CHS/ga1-3/ExenA (designated LA)
B line:
Genotype PAP1/tt4/nii-CHS/ga1-3/ExenB (designated LB)
The
seed concentration is chosen to be 3000 seeds pr m2. These plant
lines are maintained on high water levels. Visual inspection
and photos are taken on a daily basis.
2. Seed
spreading and maintenance of growth using the water pump system
Goal:
To establish a method of dispensation of the seeds and maintain
growth in a typical grid used in actual de-mining projects.
Procedure:
Firstly, pelleted wildtype seeds (not genetically engineered)
will be sprayed onto an area using a pump that works together
with the water supply. The size of the specific area is estimated
to 125-250 square meter, which is dependent on the actual amount
of pelleted wild type seeds available, and the seed concentration
per square meter. Secondly, non-pelleted wild type seeds in
a mixeture of Driwater® will be sprayed onto a minor area to
test whether the pelleting process may be substituted with the
use of the Driwater®. The growth of these plants will be maintained
using the pump and the watering system throughout the test.
3. Seed
density test
Goal:
To determine the minimum seed concentration and the following
density of plants on a square meter.
Procedure:
The concentration of seeds (# seeds/sqm) and the following density
of plants are tested. Wild type (non-genetically engineered)
plant lines are used for this experiment. The wild type plant
line Col-0 is grown in 5 squares (of 1 sqm each).
The
5 different seed concentrations within these squares are given
as follows:
" 1000 naked Col-0 seeds on a sqm (not pelleted)
" 1000 pelleted Col-0 seeds on a sqm
" 2000 pelleted Col-0 seeds on a sqm
" 3000 pelleted Col-0 seeds on a sqm
" 4000 pelleted Col-0 seeds on a sqm
4. Background
physiological measurements
Goal:
To determine the background physiological parameters for the
testing Procedure: The following parameters are measured at
09.00 am and at 09.00 pm every day):
" Air
temperature
" Soil temperature
" Air humidity
" Soil humidity
" PAR (solar radiation without UV)
5. Examination
of the herbicide concentration on local plant growth
Goal:
To determined the effect of the herbicide concentration on the
local plant population.
Procedure:
The local plant vegetation is observed when arriving at the
test site, where after the specific procedure for this test
is designed. Dependent on the observations made at the site,
small squares containing local plant vegetation will be marked
on the site. These squares will be treated with different concentrations
of Basta (10 mg/L, 20 mg/L, and 30 mg/L). Furthermore, the plant
lines growing in the test lanes (LA or LB) are tested for the
ability to grow in such areas treated with Basta. Thus, LA or
LB will be grown in some of these specific squares after the
Basta treatment, in order to observe growth of these plant lines
in competition with potential local plant vegetation naturally
resistant to Basta.
6. Growth
experiments with different ecotypes
Goal:
To examine the growth ability of several ecotypes of Arabidopsis
thaliana in order to identify superior ecotypes when growing
in the African soil. This knowledge is very important in order
to determine which ecotype that is most suitable for this technology
(most adaptable to the African growth conditions, growth rates,
etc.).
Procedure:
Growth of the following ecotypes is examined:
Ecotype: Designated:
" H. Tsukaya (NASC stock # N3963) Jap
" Ita-0 (NASC stock # N1244) Ita-0
" India (NASC stock # N9030) Ind
" Santa C. (NASC stock # N8069) S.C.
" Ler Ler " Col-0 Col
The
germination rates and biomass measurements are determined, as
well as the water requirements by varying the amount of water
added per week in the following concentrations (0, 1, 2, 3.5,
7 l water per week, respectively). Hence, this test requires
30 squares (6 ecotypes x 5 different water concentrations) of
1 x 1 meter, where 81 L of water is added per week in total.
7. Growth
experiments with different mutant plant lines
Goal:
To examine the growth ability of these two naturally selected
mutant plant lines of Arabidopsis thaliana in order to examine
the effect of the specific mutation on the overall growth performance.
The germination rates, the biomass measurements, and the water
requirement serve as scientifically controls, which are very
important if the plant lines in the test lines (see 1.) are
not growing properly.
Procedure:
Growth of the following mutant plant lines is examined (in addition
to the Col-0 laboratory wild type plant line serving as a control):
Mutant:
Designated:
" tt4-58 stop codon in CHS tt4
" ga1-3 GA deficient mutant ga1
The
germination rates and biomass measurements are determined, as
well as the water requirements by varying the amount of water
added per week in the following concentrations (0, 1, 2, 3.5,
7 l water per week, respectively). Hence, this test requires
15 squares (2 mutant plant lines and the Col-0 plant line x
5 different water concentrations) of 1 x 1 meter, where 40,5
L of water is added per week in total.
8. Growth
control experiments with different genetically engineered plant
lines
Goal:
To examine the growth ability of the genetically engineered
plant lines of Arabidopsis thaliana in order to examine the
effect of the introduced traits on the overall growth performance.
The germination rates, the biomass measurements, and the water
requirement serve as scientifically controls, which are very
important if the plant lines in the test lines (see 1.) are
not growing properly.
Procedure:
Growth of the following genetically engineered plant lines is
examined:
GMO lines: Designated:
" F3 genotype pap1/tt4/ F3
" Line 5 genotype pap1/tt4/nii-CHS L5
" A line genotype pap1/tt4/nii-CHS/ga1/ExenA LA
" B line genotype pap1/tt4/nii-CHS/ga1/ExenB LB
The
germination rates and biomass measurements are determined, as
well as the water requirements by varying the amount of water
added per week in the following concentrations (0, 1, 2, 3.5,
7 l water per week, respectively). Hence, this test requires
20 squares (4 plant lines x 5 different water concentrations)
of 1 x 1 meter, where 54 L of water is added per week in total.
Since this experiment is carried out with the plant lines F3
and L5 that are not deficient in ga1, it is decided to let these
two specific plant lines grow under net protection in order
to eliminate the risk of spreading genetically engineered seeds
to the environment.
Risk
assessment of the field trial
The
initial risk assessment of the Angola field trial experiment
should be based on what is expected to occur and what could
reasonably be expected during the trial. If a result occurs
that is not expected, then it would be necessary to review the
risk assessment. The risk assessment is written such that it
is understandable to persons skilled in the art of molecular
biology, such as an HSE inspector or individual laboratory worker.
Generic
risk assessments (such as we have for some plant species including
Arabidopsis thaliana - see information below about Thale cress)
are acceptable for the wild type plants included in the Angola
test. Relevant issues are assessed in relation to the GMO (Gene
Modified Organisms) plants used in the Angola test.
Information
about Thale cress (Arabidopsis thaliana)
Arabidopsis
thaliana is part of the natural environment in Angola, so the
testing in Ondjiva does not involve introduction of a foreign
plant specie. Arabidopsis thaliana is a cruciferous plant and
probably contains several glucosinolates that are deleterious
if consumed in large volumes under certain conditions. However,
other cruciferous plants such as cabbage and mustards are eaten
in large amounts without deleterious effects. These compounds
are products of complex secondary metabolic plant biosynthetic
pathways and it is therefore extremely unlikely that such products
could arise as a result of cDNA cloning in bacterial hosts (Keller
et al. 1978).
Why
is it necessary to follow up the trials under closed conditions
in the greenhouse with trials in open conditions?
The
greenhouse in effect provides a protected environment. The plants
react differently when exposed to wind, rain and to natural
pathogens. Thus, closed greenhouse conditions actually favoured
the growth of a colonizing fungus, while the vegetation hall
completely prevented infection with the fungus even in the non
transgenic controls. Therefore field conditions cannot be replicated
by any means at our disposal.
Description
of the inserts (transgenes)
See
Appendix A for a detailed description of the genetically engineered
plant lines.
Environmental
risk assessment
The
environmental risk assessment is carried out collectively, since
all plants included in the Angola test are Arabidopsis thaliana.
Hence, containment of the testing is explained by giving answers
to a number of questions, and providing background material
related to these questions.
Which
non-target organisms could possibly be effected?
Non-target
organisms include various ground bacteria, other useful fungi
(mychorrhiza), as well as a types of flea, flea beetle, springtail,
lice and leaf beetles. To date we have been unable to establish
a difference between transgenic and non transgenic controls.
Insofar as we can judge, these plants do not therefore pose
an ecological risk.
Prevention
of the spread of genetically modified material in the course
of this study From a purely scientific point of view we can
assume that genetic material will not be released from the transgenic
plants since Arabidopsis is strictly self-pollinating, the ecotype
chosen (labaratory strain which may not be as adaptable to the
growth conditions in Ondjiva as the native ecotype), and the
ga1-3 mutation carried by the plants. However, in view of the
public's reservations on this issue, we intend to coverthe transgenic
plants not carrying the ga1-3 mutation in the 1x1 m 2 transgenic
control squares with small pollen-proof tents to prevent the
escape of pollen and cross-pollination by insects in the test
period.
Even
though the majority of these experiments are carried out with
non-genetically engineered plant lines (ecotypes and mutant
plant lines), Aresa will make sure that a complete and thorough
clean-up procedure will be followed. The clean-up procedure
will include removal of all plants by several methods such as
manual harvesting of living plant material (also needed for
biomass determination), the use of herbicides, and the use of
a weed burner. Hence, no plants are left when leaving the test
site.
All
plant residues that are harvested during the clean-up will be
burned on a fire for complete destruction.
To
which plants could the transgenic Arabidopsis conceivably transfer
its genetic information?
The
transgenic Arabidopsis could transfer its genetic information
to other Arabidopsis plants within a radius of two metres of
the plant. In Angola this could occur likewise with Arabidopsis
and a number of wild species of the family Brassicaceae. The
genus Arabidopsis contains about 10 diploid species, such as
Arabidopsis lyrata and A. halleri. More distantly related is
a diverse group of North American N = 7 "Arabis" (now re-classified
as genus Boechera), centered in western North America. Koch
et al (2000) estimated that the genus Arabidopsis diversified
about 5 million years ago, and Boechera has been separated from
the Arabidopsis lineage for about 10 million years. Finally,
there are agriculturally important Brassicas, and the Arabis
alpine group across Eurasia.
[Figure
1 was not made available.]
Figure
1: Strict consensus tree of 10 most parsimonious trees from
Fitch parsimony analysis based upon Chs sequences. Bootstrap
support (above branches) and decay indices (below branches)
are indicated. From Koch et al, 2000.
Plants
resulting from a theroretical outcross are, however, not necessarily
capable of survival. Partly due to the ecotype (the laboratory
strain Col-0) and the ga1-3 mutation, it is almost impossible
that the transfer of genetic information to normal diploid wild
plants would produce plants capable of survival. Furthermore,
the trial area is located at a considerable distance from other
Brassicaceae fields (rape and mustard).
Additional
genetic markers were introduced to the plant lines along with
the genetic origins of interest.
Since
the advent of genetic engineering, researchers have used antibiotic
resistance genes as selective markers for the process of genetic
modification. The concern has been raised that the widespread
use of such genes in plants could increase the antibiotic resistance
of human pathogens. As is common in research, the transgenic
Arabidopsis plants are prototypes. As well as the Pap1, CHS,
ExenA and ExenB gene, which is of interest to us, the plants
also contain three markers for technical reasons.
Hygromycin,
kanamycin are antibiotics and phosphinotricine (Basta) an herbicide
used in selecting the transgenic plant lines. This gives rise
to the general public's fear that a ground bacterium could acquire
the antibiotic selection marker (gene) from the plant, and subsequently
transfer it horizontally to a bacterium that poses a risk for
humans. This bacterium could then no longer be treated with
this particular antibiotic, which is of course undesirable.
The bacterium could however be treated with a different antibiotic.
Assessment
of antibiotic resistance
The
original and most widely used selectable marker is a bacterial
gene for neomycin phosphotransferase (NPTII), an enzyme that
inactivates a number of related antibiotics including kanamycin.
After introduction of constructs containing the NPTll gene into
plant cells, kanamycin is applied to kill untransformed tissue.
Transformed cells expressing NPTII are protected from the effects
of the antibiotic and, using appropriate cell culture media,
can regenerate into whole transgenic plants. A number of other
systems employing antibiotic resistance as a selectable marker
are employed in the production of genetically modified plants.
These include the aad gene for streptomycin and spectinomycin
resistance and the hpt gene, which confers resistance to hygromycin.
Numerous studies have suggested that the presence of any of
these antibiotic resistance genes in any crop or crop products
will have negligible impact on food safety. The scientific evidence
for this conclusion is summarised briefly below.
A possible
concern about the use of antibiotic resistance as a selectable
marker is the potential to compromise the use of antibiotic
used in human (and animal) therapy. The possible transfer of
resistance to gut microorganisms and the potential for transfer
of resistance to potentially hazardous microorganisms has been
considered, as has the presence of the gene product in food
or feed. It has been concluded from extensive experimentation
that the potential for compromising the efficacy of kanamycin
or neomycin for therapeutic use in humans or animals by consuming
the food and feed products are derived from genetically modified
plants containing them is effectively zero because:
The
transfer of the NPTll gene from plant material to gut microflora
is extremely unlikely because there is no evidence that such
transfer can occur. This conclusion is supported by studies
that demonstrated that horizontal gene transfer from plants
to microbes did not occur under a variety of test conditions
(Nap et al., 1992; Redenbaugh et al., 1994; Schloter et al.,
1995; Prins and Zadoks, 1994). Moreover, there are numerous
barriers in the gut, which make this event extremely unlikely
to occur and the event, if it were ever to occur, would be unlikely
to be maintained in the absence of constant selection pressure
for resistance due to the extremely limited use of these particular
antibiotics.
In the
unlikely event of a transfer occurring by an unknown mechanism
from the genome of genetically modified plants or products derived
from them to gut microflora and this event being maintained,
this would not add significantly to the inherently large microbial
population of kanamycin and neomycin resistant microbes in the
gut of either humans (Shaw et al., 1993; Levy et al., 1988;
Nap et al., 1992; Kelch and Lee, 1978) or animals (McAllan et
al., 1973; Nap et al., 1992). The expression of the NPTll gene
in genetically modified plants is controlled by a plant specific
promoter, which is not expected to function in bacteria.
In the
unlikely event of transfer of the NPTll gene and stable propagation
of the intact gene fragment in bacteria, the gene is unlikely
to be expressed and even less likely that a DNA rearrangement
event occurs that places the functional NPTll -encoding open
reading frame in front of a bacterial promoter. Even if expressed
in intestinal bacteria, antibiotic therapy would not be compromised
as the co-factors necessary for the enzyme to inactivate kanamycin
and neomycin are not present at the required concentration range
in the gut. Moreover, the NPTII protein would be rapidly degraded
in the gut.
There
is limited therapeutic/veterinary usage of kanamycin and neomycin.
Thus, it can be concluded that the potential for the transfer
of the NPTll gene from the genome of genetically modified plant
material to microbes in human or animal gastrointestinal systems
is insignificant. There is no evidence that horizontal gene
transfer from plant cells to gut microflora can occur. In the
unlikely event of NPTll being transferred to a gut microbe,
no protein would be produced from the gene as the plant promoter
would not be expected to function in bacteria. For the antibiotic
resistance-conferring protein to be expressed, a rearrangement
of the bacterial DNA would have to occur that places the functional
NPTll-encoding open reading frame proximal to a bacterial promoter.
As this event is very unlikely to occur, potential concerns
of transformants gaining a selective advantage over other gut
microbes through the expression of an acquired antibiotic resistance
trait are further reduced.
The
safety of the NPTII protein was established as it has been shown
that nucleic acids are rapidly degraded in simulated gastric
and intestinal fluids (McAllan et al., 1973; Nap et al., 1992).
Furthermore,
the NPTll gene occurs ubiquitously in nature and resistance
to this class of antibiotics is already widespread. Therefore,
in the highly unlikely situation that a transfer event did occur
in gut microflora in humans or animals, this would not significantly
impact the overall frequency of kanamycin or neomycin resistant
bacteria in the gut or rumen. Therefore, the overall risk is
considered to be effectively zero and the therapeutic use of
antibiotics in humans or animals will not be impacted by the
commercialization of transgenic crops containing antibiotic
resistant selectable marker genes.
Assessment
of herbicide resistance
While
antibiotic resistance genes continue to be used as a selective
agent for the generation of a wide range of transgenic plants,
resistance to specific herbicides may also provide an effective
means for plant selection. In many cases herbicide resistance
genes have in fact provided a more effective system for plant
transformation (Chandler, 1995). For example, in legume species
such as peas, luoomo and lupina, herbicide resistance has proved
to be a useful selectable marker for plant regeneration (Schroeder
et al., 1993).
Herbicide
resistance marker genes may provide considerable advantage over
antibiotic resistance genes in cases where either high levels
of the antibiotic may interfere in the plant regeneration process
or where plant tissues may exhibit a high level of intrinsic
resistance. In addition, cell death in the presence of herbicide
is generally more rapid and complete, thus providing more efficient
selection. The presence of the herbicide resistant trait in
transformed plants also provides a convenient and easily assayable
system whereby the transgenic material can readily be identified/screened
using simple techniques such as leaf painting.
Genes
conferring resistance to a number of herbicide groups including
the triazines, the sulfonylureas, bromoxynil, glyphosate and
phosphinothricin are readily available. Of these, the bar gene
isolated from Streptomyces hygroscopicus (Thompson et al., 1987)
has been widely used as an effective selectable marker in the
presence of the herbicide phosphinothricin (PPT). The enzyme
inactivates phosphinothricin by the addition of an acetyl group
from acetyl coenzyme A. This gene is freely available for research
purposes and has proved particularly useful in cereals and grasses
(Vasil et al., 1993; Wilmink and Dons, 1993), as well as in
legumes.
While
the introduction of herbicide resistant genes into specific
target crops is often a major objective of plant biotechnology
programs, the extensive use of such genes as selectable markers
does, however, have some limitations. A number of potential
problems can be identified, in particular, the presence of herbicide
residues in the crop or food products derived from it. Other
issues associated with herbicide resistance may also prove to
be significant (Huppatz et al., 1995). Of these, the possibility
of the herbicide resistant biotype itself becoming a weed and
the chance of gene transfer between the herbicide resistant
crop and weedy relatives are often cited as potential problems.
However, herbicide resistant plants growing as volunteers within
a subsequent rotational crop could generally be eliminated by
treatment with a herbicide from a different chemical family.
While gene transfer between a crop and a closely related weed
with which it can interbreed is possible, astute management
should eliminate any such risk.
Of perhaps
greater significance are regulatory issues, particularly the
use of herbicide resistant genes as selectable markers in crops
where there has been no registration for use of that particular
herbicide. Although the presence of herbicide resistance as
a selection tool may not be the primary purpose for the generation
of the transgenic crop, it nevertheless may be tempting to exploit
the presence of a herbicide resistance gene as part of a weed
control strategy. Herbicide manufacturers may well be reluctant
to allow commercialization in such circumstances, particularly
with the risk of litigation should the herbicide be used outside
the regulatory framework.
In our
opinion the use of antibiotic resistance in prototypes in the
context of small, strictly supervised experiments poses no additional
risk. This type of decision always represents a political compromise
between the various parties involved. These parties represent
differing interests which naturally are coloured by ideological
differences. Therefore such decisions do not so much reflect
scientifically based concerns as the power relations in the
various decision making bodies. Such decisions do not therefore
necessarily imply an actual risk as the public may imagine.
As the
lifting of what was to all intents and purposes a moratorium
by the EU demonstrates these decisions are subject to change.
Deficiency
in production of the growth hormone gibberellin (ga1-3 mutation)
The
plant has been modified by eliminating the growth hormone, gibberellin,
encoded by the GA gene (Koornneef M, van der Veen JH, 1980).
Since the plant line for detection of landmines does not contain
a functional GA biosynthetic pathway, the plant is not able
to germinate and to set seeds making the plant male sterile.
Hence
growth of the plant lines requires addition of gibberellin in
the initial phase of vegetative growth, which exhibits a practical
control mechanism. Without addition of gibberellin, the plant
will not grow, and hence, undesired spreading of the plant is
avoided.
Scientific
background for the ga1-3 mutation
The
most dramatic phase change that flowering plants undergo is
the transition from vegetative to reproductive growth. For this
transition to be successful, plants must integrate a variety
of environmental signals with endogenous cues, such as plant
age (Bernier 1988).
In the
facultative long-day plant Arabidopsis, the transition to reproductive
growth occurs rapidly in long days but much more slowly in short
days. Several flowering-time mutants, in which the timing of
this transition is changed, have been isolated.
Analysis
of the responses of different mutants to the environment together
with studies of their genetic interactions have resulted in
a two-pathway model showing how the transition to flowering
is regulated (Martinez-Zapater et al., 1994; Weigel, 1995; Peeters
and Koornneef, 1996). According to this model, long days induce
flowering via a facultative and fast pathway, whereas under
noninductive photoperiods, an autonomous and much slower pathway
is rate-limiting. The latter pathway is thought to be related
to plant age.
The
gibberellin (GA) class of plant hormones plays a role in many
processes during plant development, including seed germination,
cell elongation, and flowering (Finkelstein and Zeevaart, 1994).
In Arabidopsis, physiological and genetic experiments have implicated
GAs specifically in the autonomous pathway of flowering. Exogenous
application of GAs accelerates flowering in wild-type Arabidopsis,
particularly in short days (Langridge, 1957).
That
there is a causal connection between endogenous GA levels and
flowering in Arabidopsis has been confirmed with several GA
biosynthesis and signaling mutants. Mutants in which GA levels
are severely reduced, such as ga1-3, are unable to flower in
short days (Wilson et al., 1992). ga1-3 mutants carry a deletion
of the gene encoding ent-copalyl diphosphate synthase (formerly
ent-kaurene synthetase A), which controls a key step in early
GA biosynthesis (Koornneef and Van der Veen, 1980; Zeevaart
and Talon, 1992; Sun and Kamiya, 1994). In long days, flowering
of ga1-3 mutants is only slightly delayed when compared with
the wild type, indicating partial redundancy of the pathway
involving GA1 under these conditions (Wilson et al., 1992; Silverstone
et al., 1997). The sequence of the ga1-3 allele suggests that
this is likely to be a null mutant (Sun and Kamiya, 1994). However,
the ga1-3 mutant contains a small amount of GA, despite the
complete absence of the ent-kaurene synthetase gene that is
required for an early step in GA biosynthesis (Zeevaart and
Talon, 1992; Sun and Kamiya, 1994)
[Page
14 in original was blank.]
References
Bernier,
G. (1988) The control of floral evocation and morphogenesis.
Annu. Rev. Plant Physiol. Plant Mol. Biol. 39:175-219.
Chandler,
S.F. 1995. The use of herbicide resistance genes as selectable
markers for producing transformed plants in Herbicide-resistant
crops and pastures in Australian farming systems, eds. McLean,
G.D. and Evans, G. (Bureau of Resource Sciences, Canberra) pp.
29- 239.
Finkelstein,
R.R., and Zeevaart, J.A.D. (1994). Gibberellin and abscisic
acid biosynthesis and response. In Arabidopsis, E.M. Meyerowitz
and C.R. Somerville, eds (Cold Spring Harbor, NY: Cold Spring
Harbor Laboratory Press), pp. 523-553.
Flavell,
R.B" Dart, E., Fuchs, R.L. and Fraley, R.T. 1992. Selectable
marker genes are safe for plants? Bio/Technology 10:141-144.
Fuchs,
R.L., Ream, J.E., Hammond, B.G., Naylor, M.W., Leimgruber, R.M.
and Berberich,S.A. 1993. Safety Assessment of the Neomycin Phosphotransferase
H (NPTH) protein. Bio/Technology 11:1543-1547.
Huppatz,
J.L., Llewellyn, D.J., Last, D.L, Higgins, T.J. and Peacock,
W.J. 1995. Development of herbicide-resistant crops - strategies,
benefits and risks m Herbicide-resistant crops and pastures
in Australian fanning systems, eds. McLean, G.D. and Evans,
G. (Bureau of Resource Sciences, Canberra) pp 15-24.
Keeler,
Richard F., K.R. VanKampen and L.F. James, eds. 1978. Effects
of poisonous plants on livestock. Academic Press, New York,
NY. xvii, 600 p.
Kelch,
W.J. and Lee, J.S. 1978. Antibiotic resistance patterns of Gram-negative
bacteria isolated from environmental sources. Appl. Environ.
Microbiol. 36:450-456.
Koch,
M., Haubold, B., Mithchell-Olds, T. 2000. Comparative evolutionary
analysis of chalcone synthase and alcohol dehydrogenase loci
in Arabidopsis, Arabis and related genera. Mol. Biol. Evol.
17 (10): 1483-1498.
Koornneef,
M., and Van der Veen, J.H. (1980) Induction and analysis of
gibberellin sensitive mutants in Arabidopsis thaliana.. Int.
Z. Theor. Angew. Genet. 58:257-263.
Langridge,
J. (1957) Effect of day-length and gibberellic acid on the flowering
of Arabidopsis.. Nature 180:36-37.
Levy,
S.B., Marshall, B., Schluederberg, S" Rowse, D. and Davis, J.
1988. High frequency of antimicrobial resistance in human fecal
flora. Antimicrobial Agents and Chemotherapy 32:1801-1806.
Martínez-Zapater,
J.M., Coupland, G., Dean, C., and Koornneef, M. (1994). The
transition to flowering in Arabidopsis. In Arabidopsis, E.M.
Meyerowitz and C.R. Somerville, eds (Cold Spring Harbor, NY:
Cold Spring Harbor Laboratory Press), pp. 403-433.
McAllan,
A.B. and Smith, R. H. 1973. Degradation of nucleic acids in
the rumen. British J. Nutrition 29:467-474.
Nap,
J.P., Bijvoet, J. and Strikema, W.J. 1992. Biosafety of kanamycin-resistant
transgenic plants: an overview. Transgenic Crops 1:239-249.
Peeters,
A.J.M., and Koornneef, M. (1996) Genetic variation of flowering
time in Arabidopsis thaliana.. Semin. Cell Dev. Biol. 7:381-389.
Prins,
T.W. and Zadoks, J.C. 1994. Horizontal gene transfer in plants,
a biohazard? Outcome of a literature review. Euphytica 76:133-138.
Redenbaugh,
K., Hiatt, W., Martineau, B., Lindemann, J. and Ernlay, D. 1994.
Aminoglycoside 3'-phosphotransferase II: review of its safety
and use in the production of genetically engineered plants.
Food Biotechnology 8:137-165.
Schloter,
K., Futterer, J. and Potrykus, 1. 1995. "Horizontal" gene transfer
from a transgenic potato line to a bacterial pathogen (Erwinia
chryanthemi) occurs -if at all-at an extremely low frequency.
Bio/technology 13:1094-1098.
Schroeder,
H.E., Schotz, A.H., Wardley-Richardson, T., Spencer, D. and
Higgins, T.J.V. 1993. Transformation and regeneration of two
cultivars of pea (Pisum sativum L). Plant Physiology 101:751-757.
Shaw,
K.J., Rather, P.N., Hare, R.S. and Miller, G.H. 1993. Molecular
genetics of aminoglycoside resistance genes and familiar relationships
of the aminoglycoside-modifying enzymes. Microbiol. Reviews
57:138-163.
Silverstone,
A.L., Mak, P.Y., Martinez, E.C., and Sun, T.-p. (1997) The new
RGA locus encodes a negative regulator of gibberellin response
in Arabidopsis thaliana.. Genetics 146:1087-1099.
Sun
T, Kamiya Y (1994). The Arabidopsis GA1 locus encodes the cyclase
ent-kaurene synthetase A of gibberellin biosynthesis. Plant
Cell 6: 1509 1518
Thompson,
C.J., Mowa, N.R., Tizard, R., Crameri, R., Davies, J.E., Lauwereys,
M. and Botterman, J. 1987. Characterisation of the herbicide-resistance
gene bar from
Streptomyces
hygroscopicus. EMBO Journal 6:2519-2523. Vasil, V., Srivastava,
V., Castillo, A.M., Fromm, M.E. and Vasil, I.K. 1993. Rapid
production of transgenic wheat plants by direct bombardment
of cultured immature embryos.
Biotechnology
11:1553-1558. Weigel, D. (1995) The genetics of flower development:
From floral induction to ovule morphogenesis. Annu. Rev. Genet.
29:19-39.
Wilmink,
A. and Dons, J.J.M. 1993. Selective agents and marker genes
for use in transformation of monocotyledonous plants. Plant
Molecular Biology Reporter 11:165-185.
Wilson,
R.N., Heckman, J.W., and Somerville, C.R. (1992) Gibberellin
is required for flowering in Arabidopsis thaliana under short
days. Plant Physiol. 100:403-408.
Zeevaart,
J.A.D., and Talón, M. (1992). Gibberellin mutants in Arabidopsis
thaliana. In Progress in Plant Growth Regulation, C.M. Karssen,
L.C. Van Loon, and D. Vreugdenhil, eds (Dordrecht, The Netherlands:
Kluwer Academic Publishers), pp. 34-42.
Declaration
of the content of this document
It is
hereby declared and confirmed that the information provided
is correct in respect to the designed testing in Ondjiva. Furthermore,
the scientifically judgement of the use of the genetically engineered
plants is to our knowledge in accordance with safety guidelines
in biotechnology. We declare that to the best of our knowledge,
having made reasonable inquiries, the information herein is
true and correct.
Notifying
Organisation's Representative:
Signature:
________________________Date: ______________________________
Printed
Name: Simon Østergaard
Project
Supervisor:
Signature:
________________________Date: ______________________________
Printed
Name: Carsten Meier
ANNEX
B:
TRIAL
INSTRUCTION - ARESA "development trial" - AVS, Sept/Nov 2003
For
these purposes a "development trial" is defined as a trial seeking
to establish whether the concept under assessment has the potential
to achieve its stated aims and whether any materials involved
are fundamentally flawed in a manner that would render it unusable
in the field. In this case it also involves gathering field
data required for further technology development. A "development
trial" may involve the assessment of parts or sub-systems that
are believed to be ready for field deployment and any such parts
or sub-systems must be identified prior to the trial and assessed
by "Acceptance Trial" criteria rather than those used for "development
trials".
This
particular trials are being conducted in co-operation with Menschen
gegen Minen (MgM), an international demining NGO with appropriate
facilities in Southern Angola. Conditions for conducting trials
at the MgM test-site at Ondjiva in Angola do not restrict the
scope and conduct of well-planned development trials. Technology
developers can observe field-conditions while retaining access
to the logistic support they need - and while living in relative
comfort.
Aim
and scope
The
aim of the ARESA development trial can be summarised as follows.
AIMS
A)
To establish whether the genetically modified plants (known
as A line: Genotype PAP1/tt4/nii-CHS/ga1-3/ExenA (designated
LA);B line: Genotype PAP1/tt4/nii-CHS/ga1-3/ExenB (designated
LB), change colour when grown above hidden landmines and UXO
in the test area.
B)
To establish whether the genetically modified plants produce
flowers.
C)
To determine the efficacy of the proposed seed distribution
system and reach a view on whether it and its ancillary parts
have the potential to achieve field utility.
D)
To determine whether either of two flowering genetic variants
have the same of better potential than the main (LA and LB)
variants.
E)
To devise and implement a watering regime on unmodified plants
to determine the minimum acceptable water requirements and also
the requirement that is optimal for fast growth.
F)
To gather data to assist in further ARESA research.
G)
To gain some field experience of the way in which Humanitarian
Demining is controlled and conducted.
H)
To facilitate the recording of a video record of the testing.
The
trial will take place over a period of six weeks (5-7 weeks)
at the dedicated MgM detector and dog test areas in Ondjiva,
Angola. Assisted by ARESA personnel and within the limitations
of restricted biotechnical knowledge, AVS will record the detailed
activities of the trial as it progresses in order to produce
an "Independent end-of-trial" report.
Constraints
and limitations
ARESA
personnel, AVS and associated visitors to the MgM site must
obey MgM camp rules and while staying in the area are requested
only to travel in company with MgM representatives.
Trial
format
The
dedicated detector test-area is already fenced. Additional mined-area
marking tape may be used to mark observation lanes throughout
the area.
The
dedicated dog-testing area is already fenced. Additional mined-area
marking tape will be used to mark observation lanes throughout
the area.
The
seeds will be broadcast using equipment brought by ARESA to
the area and by hand. Some seed in will be pelleted in one of
two growth mediums, others will not.
Two
one metre square areas will be sown with variant modified seed
and grown under pollen-proof netting.
The
observation lanes will be used to provide access for daily watering
using regimes designed to give ARESA the data required to determine
optimum water needs and also to allow close inspection without
damaging the plants.
The
observation lanes will be used to record the plant progress
three times a day throughout the trial. A photographic record
will be kept by ARESA personnel.
By
agreement, and because MgM is not assessing the technology,
the positions and identity of targets (ERW) buried in the detector-test
area has already been made known to ARESA. (This is a "Development
trial", not an "Acceptance Trial".) Details of the lane preparation,
sifting, drainage and depth are documented separately. Similarly,
details of the targets, their depth and their positioning are
documented separately.
Criteria
AVS
has not been asked to develop trial criteria. To develop detailed
judgment criteria without greater knowledge of biotechnology
would not be appropriate. However, AVS has been asked to comment
on the outcome and will do so with reference to the following:
1.
A perceived change of colour occurring above buried ERW.
2. The consistency of any colour change with reference to the
specific ERW beneath.
3. Whether or not the G3 modification flowers.
4. Whether any random colour changes occur without the presence
of ERW.
5. The practicality of the deployment method in the field.
6. The apparent professionalism with which the trial is conducted
and records are kept.
Methodology,
evaluation and acceptance criteria are to be determined and
judged internally by ARESA, but may be commented on by AVS when
deemed appropriate. AVS will advise on aspects where he feels
qualified to comment and that advice may be recorded in his
report for ARESA.
AVS
will also comment on any safety aspects that arise and reserves
the right to withdraw if activities that he considers to be
physically or ecologically irresponsible or unsafe occur.
Execution
The
trial schedule is documented by ARESA. The AVS representative
at the test site is Andy Smith. MgM staff may assist if asked,
but no such arrangement has been made in advance. The ARESA
staff present will be Anders Søndergaard and Carsten Meier.
The person with overall responsibility for safety will be the
MgM camp manager, Ken O'Connell or his deputy when he is absent.
It
is anticipated that two personnel from the film Company Bastard
will also be present throughout the trial.
The
trial will take place in dedicated test areas prepared for testing
detection technologies and Explosive Detection Dogs (EDDs) on
the outskirts of the town of Ondjiva in Southern Angola. The
trial personnel will be accommodated in a well-appointed hotel
200 meters away from the MgM camp.
There
is no anticipated explosive risk during the trials.
Annex
C: Pre-Trial Assessment - Andy Smith 30/09/03
C1.
Introduction
ARESA
plan to carry out a number of plant-growth tests and data-gathering
exercises in the MgM campsite and test-area at Ondjiva, Angola.
The plants to be used are all variants of Arabidopsis thaliana,
which is also commonly known as "Thale cress". Plants of this
type are known to occur naturally in the area, which has given
rise to concerns about the escape of genetically modified material
- see Section C2, Pre-Trial Risk Assessment.
The
author of this report is not a biologist, so cannot comment
in detail on the accuracy of scientific material presented to
MgM and the Angolan authorities by ARESA.
Testing
will be of the growth and colour-change of two genetically engineered
plant lines in the "detector-test area". This will occur alongside
a number of growth experiments on different ecotypes or mutant
plants in the "dog-training area". The later should allow the
identification of an ecotype best suited to the growth conditions
in that geographical location.
C1.1.
Detection of ERW in the detector test-area
The
stated goal of this test is to determine the efficacy of the
genetically engineered plant lines in terms of both growth and
reaction to the presence of buried ERW.
The
seven test lanes will be sown with a mixture of pelleted seeds
of plant lines LA and LB, which have a minor genetic variation.
A line:
Genotype PAP1/tt4/nii-CHS/ga1-3/ExenA (designated LA)
B line:
Genotype PAP1/tt4/nii-CHS/ga1-3/ExenB (designated LB)
The
seeds will be spread as a concentration of 3000 seeds per square
metre and they will be watered regularly. Visual inspection
and a photographic record will be made by ARESA daily.
Two
small areas within the detector test area will also be used
to determine the growth of the LA and LB seed types in the presence
of a specific herbicide that they are modified to resist.
AVS
comment: care must be taken not
to overspread the seeds and any subsequent growth medium because
the area backs onto a public path, separated by a chain-link
fence. No overspray into a public area should occur.
AVS
recommends marking access paths between the test-lanes to facilitate
observation and maintenance access without damaging plants.
C1.2.
Seed and water broadcasting using a water pump system
The
stated goal of this test is to evaluate a proposed method of
spreading seeds and water that is intended for practical field
use. The pump will not be used to broadcast modified seed, so
allowing assessment without risk of uncontrolled dispersal of
GM seed.
AVS
comment: ARESA should be aware that manual demining does
not normally make use of a "grid system". Some Humanitarian
Demining organisations use EDDs in this way.
C1.3.
Seed density (data gathering exercise)
The
stated goal of this test is to determine optimum seed concentrations
to achieve good coverage and the variation in plants per square
metre that results from varying the seed concentration. The
seeds used for this experiment will not be genetically modified,
and the test will occur in discrete sections of the dog-test
area. Five separate square metre areas will be used. The five
variations of seed deployed will differ only in whether they
are pelleted in a growth medium and in the density with which
they are sown.
AVS
Comment: While this test does not involve GM seed, it
does involve sowing seeds from varied geographical locations
around the world. AVS recommends that these plants be destroyed
as soon as flowers start to appear (first buds form).
C1.4.
Measuring context variables (background physiological measurements)
The
stated goal of this data gathering exercise is to record the
following variables at the site, so providing a context within
which to view the test results. The exercise involves recording
the following data at 09.00 am and at 09.00 pm each day:
"
Air temperature
" Soil temperature
" Air humidity
" Soil humidity
" PAR (solar radiation without UV)
C1.5.
Applying herbicide concentrations to local plant growth
The
stated goal of this data-gathering exercise is to determine
the effect of the herbicide concentration on the local plant
population. Small areas (single square meters) of local plants
and the plants in the detector test-area will be treated with
varied concentrations of the herbicide "Basta".
AVS
comment: the purpose of this test is not outlined in
the ARESA documentation but is believed to be to indicate whether
spraying Basta in a particular concentration could kill local
plants while allowing the genetically modified plants (LA and
LB) to grow. This could allow the seeds to be disseminated over
a suspect area without cutting the undergrowth, so saving time
and cost.
C1.6.
Growth experiments with different naturally occuring types of
"Thale cress"
The
stated goal of this data-gathering exercise is to examine the
growth of several types of the ""Thale cress" plant with the
aim of identifying whether any one grows better in the local
soil.
The
types or "Ecotypes" to be used are:
" H.
Tsukaya (NASC stock # N3963) Jap
" Ita-0 (NASC stock # N1244) Ita-0
" India (NASC stock # N9030) Ind
" Santa C. (NASC stock # N8069) S.C.
" Ler Ler
" Col-0 Col
The
germination rates and biomass achieved within a timeframe will
be measured, and the water requirements of each type assessed.
These experiments will require 30 x 1 metre squares (6 ecotypes
x 5 different water concentrations), with a total of 81 litres
of water added each week.
AVS
Comment: While this test does
not involve GM seed, it does involve sowing seeds from varied
geographical locations around the world. AVS recommends that
these plants be destroyed as soon as flowers start to appear
(first buds form).
C1.7.
Growth experiments with genetically modified variants
The
stated goal of this data-gathering exercise is to examine the
growth ability of two "mutant" plant lines of Arabidopsis thaliana
("Thale cress") in order to examine the effect of the mutations
on the overall growth performance. Germination rates and biomass
achieved within a time frame will be measured, and a record
of the water applied will be made. These experiments could prove
crucial if the genetically modified plants in the detector test-area
do not grow as anticipated.
Details
of the growth of the following mutant plant lines will be gathered
along with a Col-0 laboratory wild plant line that serves as
a "control". This data-gathering will take place over 15 discrete
square metres. The two GM plant types and the Col-0 plant line
will each be in five separate square metre plots receiving five
5 different concentrations of water. In total, 40.5 litres of
water will be added each week.
The
genetically modified plants to be used are designated as:
"
tt4-58 stop codon in CHS tt4
"
ga1-3 GA deficient mutant ga1
AVS
Comment: it is hard for me to see how this data-gathering
exercise varies from C1.8 below. AVS recommends that these plants
be destroyed as soon as flowers start to appear (first buds
form).
C1.8.
Growth control experiments with other genetically modified plant
lines
The
stated goal of this data-gathering exercise is to examine the
growth ability of four other "genetically engineered" plant
lines in order to examine the effect of the modifications on
their overall growth performance. Germination rates and biomass
achieved within a time frame will be measured, and a record
of the water applied will be made. These experiments could prove
crucial if the genetically modified plants in the detector test-area
do not grow as anticipated.
Details
of the growth of the following modified plant lines will be
gathered. This data-gathering will take place over 20 discrete
square metres. Each of the four plants types will have five
separate plots, each receiving a different concentration of
water each week.
The
genetically modified plants are designated as:
"
F3 genotype pap1/tt4/ F3
" Line 5 genotype pap1/tt4/nii-CHS L5
" A line genotype pap1/tt4/nii-CHS/ga1/ExenA LA
" B line genotype pap1/tt4/nii-CHS/ga1/ExenB LB
The
F3 and L5 variants are not modified in a way that will stop
them flowering and producing pollen, so their plots will each
be covered by "pollen-proof" net protection.
AVS
Comment: it is hard for me to see how this data-gathering
exercise varies from C1.7 above. There appears to be no research
advantage in allowing these plants to flower. AVS recommends
that these plants be destroyed as soon as flowers start to appear
(first buds form)
C1.9
AVS recommendations
If the
plants "will react on NO2-groups, which are cleaved off from
the explosive molecules that leak out of the buried landmine",
it would seem to a layman to be appropriate to test whether
the plant also reacted to the nitrate residues left when common
nitrate fertilisers had been applied to the land in previous
years.
It should
be noted that the presence of nitrate fertilisers could falsify
the results - and so some kind of pre-test assessment of their
presence in the detector test-area would seem desirable.
C2.
Pre-Trial Risk Assessment (AVS)
AVS
(Andy Smith) has no background in biotechnology and cannot assess
the ARESA Risk Assessment for completeness or consistency. That
said, the planning documentation has given rise to the observations
and recommendations below. Risk Assessments are intended to
predict things that could go wrong, and take measures to minimise
the chance of that happening. This is what I have tried to do
in what follows.
I
identify three kinds of risk in what follows. These are:
1. The risk of failing to collect the required test-data.
2. The risk of releasing GM material into the environment.
3. The risk of an unintended detonation or fire during burn-off.
The
first of these can be minimised procedurally following the advice
under C2.1 and C2.4 below. The fear of the second can be minimised
using the recommendations under C2.2 and C2.3 below, but AVS
is not qualified to assess how significant any residual risks
may be. The third of these perceived risks is negligible and
can be reduced further if the recommended measures under C2.3
below are followed.
C2.1
Complexity and human error
The
series of experiments, tests and data-gathering exercises planned
by ARESA are complex, and that complexity introduces risks of
human and "nuisance" error. Human error is self explanatory.
"Nuisance" error in this instance can be defined as the intended
or unintended disturbance/removal of some or all marking systems
(by strong winds, animals, birds, etc).
By
my calculation, eight variant seed types that are genetically
modified or "mutant" (I am not sure of the difference) and five
naturally occurring seed types are to be used over a total of
more than 74 discrete areas.
The
74 areas are:
Detector
test area: two GM seed variants plus two areas sprayed with
herbicide = 4 discrete areas.
Dog-training
area: six GM/mutant seed types and one control, each in five
discrete areas = 35 discrete areas.
Plus
six naturally occurring seed variants each in five discrete
areas = 30 discrete areas.
Plus
five discrete square metres of seed-density variation exercises
= 5 discrete areas.
Plus
an uncertain number of herbicide exercises on naturally occurring
growth.
There
is a very real potential for confusion over labelling and mapping
which area is which. Not only could this render the results
of all data-gathering exercises uncertain, I believe that it
could also be perceived as hazardous if the genetically modified
plants are allowed to mature and flower.
AVS
Recommendation
A complete map of the testing area should be drawn prior
to spreading any seed. When the map has been approved by MgM
(giving permission to use that ground) the areas must be clearly
marked out using stakes and marking tape. The stakes used in
those areas with genetically modified/mutant seed must be painted
a different colour to those with naturally occurring seeds.
Each discrete area must be labelled with a durable label BEFORE
seeds are spread (so helping to ensure that the right seeds
are spread in the right places). The stakes used to mark the
areas where the two GM seed-types that are expected to produce
flowers will be sown should be a third colour - so making it
easy to immediately identify these areas if the pollen-proof
netting is disturbed for any reason.
C2.2
Continued concern about pollen escapes
The
most important reason for ARESA's presence in Ondjiva is the
performance testing of the two genetically modified plants that
will be used in the "detector test-area" where there are some
items of ERW with their original HE content.
ARESA
state that "the triggered colour change of the plants will occur
in the vegetative stage of plant growth, which enables identification
of the specific plants growing in the near by presence of landmines
within 3-4 weeks".
My
understanding is that these plants are also effectively "neutered"
and cannot produce seed that would survive. The GA-13 modification
is intended to prevent flowering, but in the ARESA assessment
it is clear that day length can over-ride this restraint and
so the plants in Ondjiva (where sun intensity and day length
have not been determined) may flower (and may even be expected
to flower).
If they produce flowers (and so pollen) not everyone would be
convinced that allowing the pollen to be transferred to similar
plant types in the area by insect activity is safe.
If
the plant flowers, it is not possible for MgM or myself to determine
whether the pollen presents a threat of successful cross-fertilisation
with local plants. ARESA identify a wide range of plants that
could theoretically be affected in their Risk Assessment. They
also state that "Plants resulting from a theoretical outcross
are… not necessarily capable of survival". This means that ARESA
recognise that they may survive.
AVS
Recommendation
Because flowering should not occur until after the "vegetative
stage" of plant growth and any colour change should have become
apparent before then, I recommend that the GM plants types in
the detector test-area be destroyed as soon as any flower buds
appear.
Given
the declared purposes of the data-gathering exercises, I further
recommend that all plant types, genetically modified, mutant
or otherwise, in the dog training-area should be destroyed when
the first buds appear in any discrete test area.
The
declared purposes of these tests do not require the plants to
flower. I include the non-modified plants in this recommendation
because we have no right to release pollen from a plant that
only naturally occurs in Japan or India with no knowledge of
how that might effect local strains - and also because the flowering
is not necessary for the successful completion of the declared
research aims. Even the most minimal risk should be avoided
if there is no advantage in taking that risk.
C2.3
Antibiotic and herbicide resistance
AVS
is not qualified to comment in detail on the accuracy of ARESA's
view that the genetic modifications and/or markers present no
risk to human or animal population if they enter the wider environment
and so the food chain. AVS does observe that ARESA's confidence
that the antibiotic resistance could not be transferred is qualified
by the declaration that any transfer would "have negligible
impact on food safety". No impact at all is publicly acceptable,
so every effort must be made to avoid it, and must be seen to
be made.
AVS
recommendation
I strongly recommend that all measures be taken to ensure
that no animals enter the test areas and that birds are discouraged
(using bird-scarers) from visiting the areas where ALL plants
are grown. Given the small potential for ground-bacteria transferring
genetic material, I recommend that the ground in the test and
training areas be processed by burning at the end of the tests,
so destroying all roots and making an effort to kill any affected
bacteria. (I do not know whether this would be effective, but
it could be done relatively easily so I recommend it.) It may
be necessary to lift some of the mines/devices in the "detector
test-area" to prevent fire damage before this is done. No mines/devices
should be lifted without express permission from MgM. All mines/devices
that contain any explosive element and are within an agreed
depth of the ground surface MUST be lifted before burning off
the area (suggested depth 10cm).
Any
ideas for inhibiting the access of small mammals should also
be pursued.
C2.4
Objectivity of the detector test area testing
There
is a risk that ARESA will not be able to convince the world
of its results when the testing and data-gathering has been
completed.
AVS
recommendation
In order to ensure that the "independent observations" that
AVS makes of the results from the "detector test-area" are considered
trustworthy by the outside world, AVS recommends that the "detector
test-area" be kept locked at all times.
Annex
D: AVS/ ARESA Biotech contract
Contract
between: Mr. Andy Smith and Aresa Biodetection Aps
Plant
technology testing in Ondjiva, Angola, 2003
Aresa
Biodetection (the company) is about to test its technology platform
at the landmine test area of Menschen Gegen Minen (MGM) in Ondjiva
located in the southern part of Angola.
The
company will employ Andy Smith (AS) on a contract basis for
this specific trip in order to fulfil the roles described below.
The
role of AS in Angola:
Observer: AS shall document the experiment carried out in Angola
by writing up a report that describes the test results achieved.
It is of high priority that the report emphasises on the sensitivity
of the plant line(s) grown in respect to the different type
of mines detected/not detected. Furthermore, the report shall
contain reflections upon observations made in respect to the
practical use of the technology (seeding, watering techniques
and systems, water requirements, plant growth, etc.) in light
of the complexity when operating in a landmine infected area.
Technical
advisor: AS shall act as an advisor on the technology in development
on behalf of his in-depth experience within de-mining. Hence,
AS shall challenge existing ways of thinking, and discuss new
ideas generated with the employees of the company.
Safety
advisor: AS shall act as an advisor on safety related issues
due to his experience from operating in landmine infected areas.
Hence, AS should guide the employees of Aresa and employees
of Bastard to behave as safely as possible in Angola. In case
of any doubt, AS should consult the MGM safety Officer Ken O'Connell
for advice on safety related matters.
Terms
and conditions:
Period:
The plant technology testing is scheduled for 6 weeks with estimated
time of departure from Europe in the beginning of October. Hence,
AS will be offered employment on a contract basis for the company
in the period of October 6 to November 16, 2003.
Working
schedule:
As a general rule, AS is hired for 5 days a week during the
period. Nevertheless, the working schedule of AS is flexible,
but AS shall aim at choosing a schedule that allows him to achieve
the best possible documentation of the testing.
Compensation:
AS will receive [excised] per week, which equals [excised] in
total for the period. Furthermore, to cover food and drinks,
AS will receive a daily allowance of DKK [excised] per day (approximately
equal to [excised] according to the exchange rate (on July 22)
of DKK 10,49 per £). If AS and the company agree to extend the
period for more than 6 weeks, AS will get paid accordingly with
a weekly rate of [excised] plus daily allowance of DKK [excised].
Payment
will occur in two separate, but equal, transactions. Half of
the compensation [excised] plus daily allowances for three weeks
will get transferred to the account of AS on October 1, 2003.
The same amount of money will be transferred to the account
of AS at the end of the period. Payment will be transferred
to the following bank account of AS: AVS Consultants Ltd., Business
Account, Midland Bank…[details excised].
Expenses:
Expenses covering visa, airfares to Namibia and back to United
Kingdom, transportation between Windhoek and Ondjiva (and back),
and hotels will be covered by the company.
Compensation
if cancellation occurs:
The testing of the technology is dependent on getting appropriate
approvals (to test the technology platform by MGM; to transfer
the genetically engineered seeds via Namibia to Angola by the
authorities; and to grow these seeds). Due to the engagement
of AS to the project, and booking of the trip, AS will receive
compensation for the full period ([excised] in total) if the
testing is cancelled before departure. AS will not receive any
kind of compensation if he cancels his engagement, or fails
to participate on the trip for personal reasons (sickness, etc.).
Confidentiality
agreement:
In order to control and protect the patent strategy and the
communication strategy of the company, AS agrees to treat all
knowledge and insight material regarding the use and the development
of the technology platform, which may come to his possession,
as strictly confidential. Hence, AS will only communicate any
information about the company and its technology to a third
party, after getting accept from the management of the company.
On behalf of the company:
Annex
E: The MgM Ondjiva Test Area
[The
original "map" of the test-area has photographs of each lane
and of each item in the lanes. The pictures have been removed
from this Annex in order to keep this report a manageable size.]
AVS
had previously constructed a small detector test area for MgM
near the town of Ondjiva in Southern Angola. It was not constructed
for the ARESA tests and so many of the "targets" concealed in
the test lanes do not contain any High Explosive. However, shallow
mines containing both TNT and RDX are present, along with ordnance
and deeply concealed AT mines.
Annex
E, part 2: Test field details
Removed
for web publication.
Annex
F: Relevant emails
-----Original
Message-----
From:
Andy Smith [mailto:mgmang10@mail.station12.com]
Sent: Friday, October 31, 2003 2:50 PM
To: 'simon@aresa.dk'
Subject: To Andy Smith

Dear
Simon,
The
attached show the plants in Lane 7 today. The arrow is pointing
to a cluster of 17. They tend to be in small clusters like this.
This shows the most advanced plants I can find. The other picture
[not reproduced] shows the local plant growth compared to the
seeded growth.
If I
do not hear from you, I will destroy them in the morning. My
phone number is 00 244 92 645083
Regards
Andy
-----Original
Message-----
From: Simon Østergaard [mailto:simon@aresa.dk]
Sent: Friday, October 31, 2003 11:57 AM
To: 'MgMAng10'
Subject: SV: To Andy Smith
Dear
Andy,
Thanks
for your mail! I hope it is not too late to comment on the development
of the plants. I'm sad to hear that you are planning on giving
up lane 7. I would very much appreciate if you could stay for
longer.
I believe
the plants should be growing as they were in the beginning of
this week, and since the second round of seeds sawed is delayed
in comparison with the first round. It is impossible to see
plant growth just by looking at these form day to day; it is
important to measure growth of the rosette by putting sticks
in the ground and observing the growth over a couple of days
(I'm aware that you may already have done this). Furthermore,
I believe that the roots should go down 5-10 cm in the soil
in order to induce the red colour change due to the extensive
watering carried out.
I hope
it is possible to motivate you to give it a further go. Let
me know if this is possible by any chance.
Best
regards,
Simon.
-----Original
Message-----
From: Andy Smith [mailto:mgmang10@mail.station12.com]
Sent: Friday, October 31, 2003 3:11 PM
To: 'Simon Østergaard'
Subject: RE: plant success....
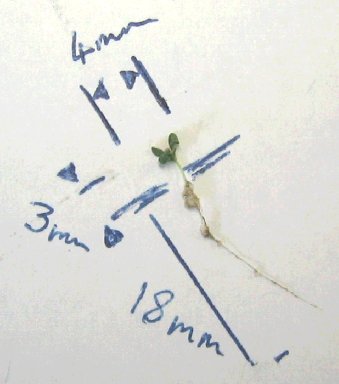
Dear
Simon,
The
attached shows the largest plant I could find in Lane 7 thirty
minutes ago. Your suggestion of placing sticks in the ground
and measuring daily growth is absurd. I admit that the root
is longer than I would have expected, but at less than two centimetres
life is just too short to wait for it to grow another eight.
I don't
know what Carsten has been telling you, but this is reality.
I expect to end the trial tomorrow.
Regards
Andy
-----Original
Message-----
From: Simon Østergaard [mailto:simon@aresa.dk]
Sent: Saturday, November 01, 2003 1:21 AM
To: 'MgMAng10'
Subject: SV: plant success....
Dear
Andy,
No doubt
it will take longer to get the roots to the desired depth than
the timeframe within we can keep you on site. Thus, we will
accept that you will be finishing up the trial tomorrow. We
appreciate your effort and that you will clean up lane 7.
Many
thanks.
Regards,
Simon
-----Original
Message-----
From: Andy Smith [mailto:mgmang10@mail.station12.com]
Sent: Saturday, November 01, 2003 7:12 AM
To: 'Simon Østergaard'; 'im@mgm.namib.com'; 'Hendrik Ehlers'
Subject: RE: Magic mushrooms in Ondjiva....
Dear
Simon,
It was
agreed with Carsten that if there was no significant increase
in the plant's size after one full week under shade netting,
the trial would end. At that time, I pointed out that the very
sparse coverage meant that even if these plants matured, there
was little chance of them doing so on top of a mine. The plants
have not grown above ground at anything remotely approaching
the rate that Carsten told me he anticipated, and have barely
grown at all despite limitless water. Fungi have been having
a wild time - with several psylopsybin in evidence, from which
I infer that the shade netting is effectively cutting out the
sunlight (this was never measured to my knowledge - so the inference
is not of much scientific value). The fungi grow more than 5cm
in a day and have withered by the following day. Funny but I
never anticipated coming to Ondjiva to grow magic mushrooms.
I now
have the problem that there have been torrential rains in the
last two days. You seem to think that I only have to worry about
Lane 7? I do not only have to clean up Lane 7, but the whole
area including those places where various seed did not germinate
but may be dormant and overspray areas. A little of this was
done on Monday and Tuesday but it takes a long time and nothing
occurred on Wednesday when I was off for the day (despite my
best efforts to ensure that it was - this is how it goes in
Africa). It is now impossible to finish the burning off process
for two reasons. The gas torch supplied produces a very gentle
flame - probably because of low pressure in the bottled gas
used for cooking here. And the ground and vegetation is waterlogged.
Burning was wanted because of dispersed GM seed that could not
possibly be seen and became mixed with dust that has now become
a surprisingly sticky mud.
The
Roundup weedkiller (restricted in Denmark) works over 10 days
and will not work at all if it rains within six hours of application
(written in the instructions). It should be sprayed using a
plastic bottle, spray and hoses - which I do not have. (The
instructions warn that using metal parts risks an explosion
of hydrogen gas.) So I would have to apply it by using a plastic
watering can. Its time-delay means that I cannot SEE whether
it is effective, and it will be ineffective if the rain continues.
(I know that the Roundup is not meant to kill the GM plants,
but do not really believe that: in any case, if I remove the
shade netting, I believe that they will die like all the others.)
The other weedkiller bought by Carsten in Namibia is an unknown
entity and would probably be banned in Europe. I asked Carsten
to find out its ground-life more than two weeks ago, and several
times thereafter. This is important because the camp uses a
borehole and I do not want to apply a restricted substance that
may contaminate a sweet water source. Carsten told me that he
had given you the list of ingredients and asked you to find
out the safe usage. Please advise whether Carsten did in fact
ask you and, if so, what the result of your inquiries was?
Regards
Andy
-----
Original Message -----
From: "Didactylos"
To: "Simon "
Sent: Friday, November 07, 2003 5:42 PM
Subject: Home
Dear
Simon,
I am
back in UK now. I burned off the Lane 7 area on Monday this
week.
Do you
want a final report or not? If so, there will be additional
costs involved. If you want copies of the photographs I took
(an incomplete record because everyone else was taking pictures
professionally) please let me know. There will, of course, be
costs involved.
Regards
to all,
Andy
-----
Original Message -----
From: "Simon Østergaard"
To: "'Didactylos'"
Sent: Monday, November 10, 2003 12:07 PM
Subject: SV: Home
Dear
Andy,
We don't
want a report and photographs for additional costs. Although
you were paid for an extra week including per day allowances,
after you cancelled the contract with Aresa, we will not ask
you to refund anything.
We consider
the collaboration as finished in relation to the deliverables
and economic transactions. Due to our conversation with Hendrik
regarding possibly continuation of the testing in the future,
we ask you to respect the confidentially agreement included
in your contract.
Best
regards,
Simon.
-----
Original Message -----
From: "Didactylos"
To: "Simon Østergaard"
Sent: Monday, November 10, 2003 1:56 PM
Subject: Re: payments
Dear
Simon,
> We
don't want a report and photographs for additional costs.
I will
finish the report for my own records - also for the MgM directors
who had most concern.
> Although
you were paid for an extra week including per day allowances,
> after you cancelled the contract with Aresa, we will not ask
you to
> refund anything.
May
I remind you that - at your request - I stayed on for a full
week after the others left. Also, I had continued to monitor
the trial before that - because MgM had made my "policing" a
condition of the trial's continuation. So I would not have been
inclined to refund anything anyway. I do not think that I owe
ARESA anything, rather - I believe that the opposite applies.
Please
send me an up-to-date email address for the BASTARD TV crew.
I have pictures of them at work which they might need. Thank
you.
Regards
Andy
See also ARESA "red plants" and the con goes on*